- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
a) Electrokinetic properties of human cells
Knowledge and understanding of the electrokinetic properties of cells makes it possible to envisage multiple applications in biomedical research, such as diagnosis or monitoring the progression of cancer. The application of non-uniform alternating electric fields in microfluidic chips, and more particularly the dielectrophoresis force, makes it possible to characterize populations of cells without specific markers.
A first fundamental study allowed us to describe the competition between dielectrophoretic and electro-hydrodynamic forces (electrothermal effects, electro-osmosis) to determine the response of human cells in such conditions [BENOIT Plos-one 2014]. The comparison of the model with the experimental observation of the movement of such cells in a microfluidic device (Fig. 5 (a,b)) comprising micro-structured electrodes was carried out. From this study, a new method for determining the cutoff frequency (limit between attractive and repulsive dielectrophoresis) of human cells on a statistical number of cells was developed.

(a) Microfluidic device (b) Experimental example of dielectrophoresis on human cells. (c-e) Determination of Clausius-Mossotti factor for different human cells: (c) different organs (prostate/kidney/blood), (d) Prostate different states (cancerous/non-cancerous) (e) Prostate different stages (increasingly more cancerous).
We then determined the cutoff frequencies of lines originating from different epithelial tissues (kidney and prostate) or from circulating cells (Fig. 5(c)). Cutoff frequencies have been shown to be statistically different between these lines. In addition, these developed methods made it possible to differentiate the dielectrophoretic signatures of cells from the same organ as in the case of the prostate which presents healthy cells and cancer cells at different stages of evolution of the cancer (Fig. 5 ( d,e)) [VAILLIER Analytical chemistry 2016] (coll. CEA/Big).
We were also interested, with our biologist partner (CEA/Big/BioMics), in understanding the mechanisms of cell polarization under alternating electric fields. We modified the cell membrane chemically or biologically to understand the molecular origin of the cutoff frequency. It has been shown that the concentration of proteins and the activity of certain ion channels significantly increase the cut-off frequency of cells [VAILLIER Electrophoresis 2015]. By exploiting the effects of dielectrophoresis on cells, it becomes possible to finely characterize their dielectric properties, and to propose new detection and diagnostic technologies in the longer term.
b) Grayscale lithography for the study of cellular mechanics
The object of this work is the study of the mechanics of adherent cells.
The objective is to use cutting-edge technological tools to shed innovative light on questions relating to the sensitivity of cell adhesion to mechanical constraints (rigidity of the extracellular environment, forces) and its consequences on cellular behavior ( migration, proliferation, differentiation). Since the 2000s, numerous studies have explored cellular responses to rigidity with culture media whose mechanical properties are uniform. Recently, by measuring the rigidity of human pituitary tissues, we showed that in vivo, cells evolve in a heterogeneous environment in rigidity, with gradients of the order of kPa/µm and textures of subcellular size (a few tens from µm to a few hundred nm) [BOUCHONVILLE Soft Matter 2016].
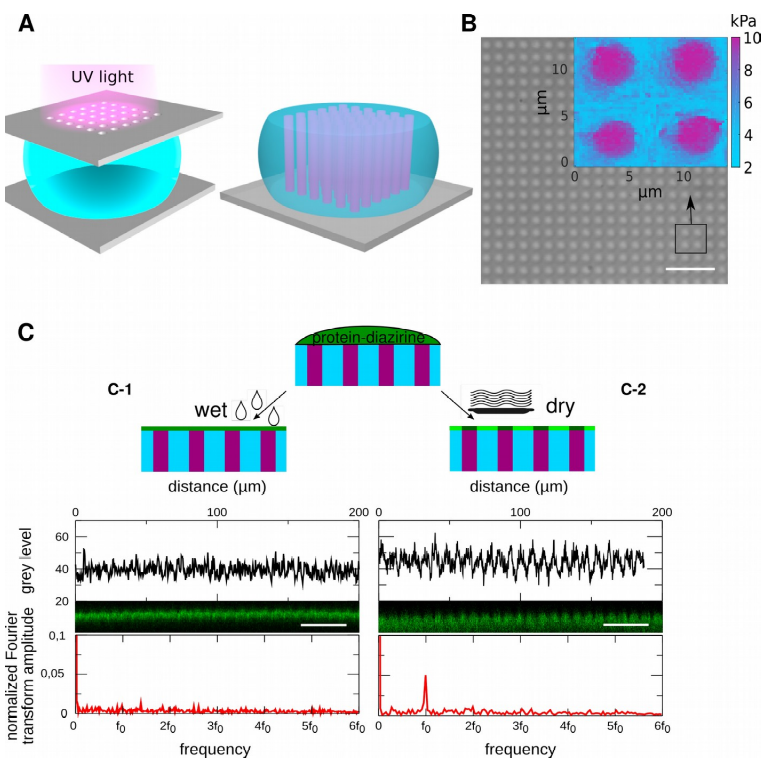
2D grayscale lithography of polyacrylamide hydrogels modulated in rigidity for cell adhesion, with tunable surface chemistry. A) Photochemistry is optimized to limit the diffusion of entities in aqueous solution and transfer micron patterns. B) Interferential contrast microscopy image of 3.5 µm plots with a period of 7.5 µm. Insert: stiffness map obtained by AFM. The pads have a rigidity of 9.1+/- 1.2 kPa, the bottom of 5.1 +/-0.9 kPa. C) The rate of surface dehydration controls the distribution of surface chemistry. C-1) maintaining surface hydration allows a uniform distribution of surface chemistry (see confocal section and Fourier transform of the intensity profile). C-2) Dehydration of the hydrogel surface leads to the concentration of the surface chemistry on the rigid pads (the Fourier transform of the intensity profile shows a periodicity of f0=1/7.5µm-1.)
In this context, we have developed culture media presenting this type of stiffness modulation, and we have developed analytical and numerical tools to measure cellular forces in response to these stiffness modulations. Finally, we set out to extend these advances to 3D geometries, to extend our understanding to three-dimensional tissues. To date, when the mechanical properties of the environment are emerging as an important parameter in tumor invasion or differentiation processes, answering these questions should in particular contribute to directing research on the mechanisms of fight against the most common cancers. more invasive.
c) Development of 2D extracellular matrices with modulated mechanical properties at the micrometric scale
The transposition of LTM’s know-how in the design and optimization of organic resins to an aqueous resin, polyacrylamide, has made it possible to develop polyacrylamide photochemistry 10 to 100 times faster than the state of the art. Thus, the diffusion of species is reduced, and we were able to reproduce rigidity patterns at the micron scale with gradients of several kPa/µm, as observed in vivo (Fig. 6) [EP2785747]. Polyacrylamide is an inert hydrogel, which requires functionalization of its surface to allow cell adhesion. For the first time, we have shown that we can freely couple or decouple surface chemistry from rigidity by varying the hydration rate of the hydrogel (Fig. 6). This new tool makes it possible to combine mechanical structuring and chemical structuring, and to independently control the chemical and mechanical environments to which cells are exposed. This innovation gave rise to 2 patents [FR1851833, FR1852101], and know-how deposited with the CNRS. All of these technological advances enabled the creation of the Cell&Soft company in 2018.
 Measurement of cellular stresses on textured hydrogels in rigidity. A) Adherent cell (Bar 20µm). B) Principle of traction force microscopy: the movement of thousands of fluorescent markers is followed and makes it possible to quantify C) the stresses exerted by the cell on the matrix (cell-to-matrix stresses) and D) the intracellular stresses ( intracellular stresses). E) The measurement of these constraints shows that the cells probe their entire surface but with constraints of greater amplitude in areas of high rigidity. F) The cells pull on the support with a force proportional to the local stiffness. G) Intracellular forces respond to the average stiffness beneath the cell and exhibit a threshold effect. (2 experiments, 12 cells, 39 measurements).
Measurement of cellular stresses on textured hydrogels in rigidity. A) Adherent cell (Bar 20µm). B) Principle of traction force microscopy: the movement of thousands of fluorescent markers is followed and makes it possible to quantify C) the stresses exerted by the cell on the matrix (cell-to-matrix stresses) and D) the intracellular stresses ( intracellular stresses). E) The measurement of these constraints shows that the cells probe their entire surface but with constraints of greater amplitude in areas of high rigidity. F) The cells pull on the support with a force proportional to the local stiffness. G) Intracellular forces respond to the average stiffness beneath the cell and exhibit a threshold effect. (2 experiments, 12 cells, 39 measurements).
Multi-scale sensitivity of cells to the mechanical properties of their environment
Mastering the fabrication and functionalization of cell culture supports exhibiting modulations of rigidity at the micron scale has allowed us to probe the scale at which cells measure the mechanical properties of their environment. Indeed, several points of view have opposed each other for around fifteen years on the mechanisms which allow animal cells to perceive the rigidity of their environment: does mechano-sensitivity take place at the scale of points of anchoring, focal adhesions which are micron assemblies of proteins, or on the scale of the actin cytoskeleton (and therefore on the cellular scale), the latter maintaining the adherent cell under tension and therefore indirectly deforming the cellular environment? To answer this question, we proposed new analytical approaches that make it possible to quantify intracellular forces [MOUSSUS Soft Matter 2014], the cellular Young's modulus, and extracellular forces in the presence of a heterogeneous mechanical environment.
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn